
Similarly, CBr 4 is used in combination with triphenylphosphine in the first step of the Corey-Fuchs reaction, which converts aldehydes into terminal alkynes. In combination with triphenylphosphine, CBr 4 is used in the Appel reaction, which converts alcohols to alkyl bromides. In fact, it is the structure in the diffuse intensity that provides the information about the details of the structure. The combination of censored Frenkel disorder and displacive disorder implies a considerable amount of disorder inside the crystal which leads to highly structured sheets of diffuse scattered intensity in X-ray diffraction. Even for the remaining combinations displacive changes occur that better accommodate neighbor to neighbor distances. This rules out certain orientational combinations when two neighbor molecules are considered. Moreover, they cannot take these orientations entirely independently from each other because in some cases the bromine atoms of neighboring molecules would point at each other leading to impossibly short distances. Recent work has shown, however, that the molecules are restricted to only 6 possible orientations ( Frenkel disorder). It was thought in the past that the molecules could rotate more or less freely (a 'rotor phase'), so that on a time average they would look like spheres. Roughly speaking, the CBr 4 are situated on the corners of the cubic unit cell as well as on the centers of its faces in an fcc arrangement. The high temperature α phase is known as a plastic crystal phase. Critical temperature is 439 ☌ (712 K) and critical pressure is 4.26 MPa. ĭue to its symmetrically substituted tetrahedral structure, its dipole moment is 0 Debye. Tetrabromomethane has two polymorphs: crystalline II or β below 46.9 ☌ (320.0 K) and crystalline I or α above 46.9 ☌. Both names are acceptable under IUPAC nomenclature. If the second element is oxygen, the trailing vowel is usually omitted from the end of a polysyllabic prefix but not a monosyllabic one (that is, we would say “monoxide” rather than “monooxide” and “trioxide” rather than “troxide”).Tetrabromomethane, CBr 4, also known as carbon tetrabromide, is a bromide of carbon. Normally, no prefix is added to the first element’s name if there is only one atom of the first element in a molecule. Table 4.1 "Numerical Prefixes for Naming Binary Covalent Compounds" lists these numerical prefixes. A system of numerical prefixes is used to specify the number of atoms in a molecule. The second element is named by taking the stem of the element name and adding the suffix - ide.

The first element in the formula is simply listed using the name of the element. Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. For example, we have already seen CH 4, the molecular formula for methane. Numerical subscripts are used if there is more than one of a particular atom.
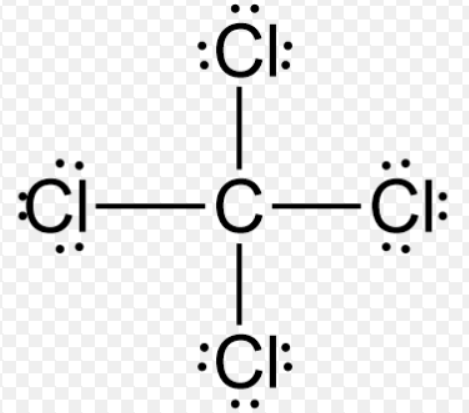

Then the other nonmetal symbols are listed. Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H 2O is the prominent exception). because these compounds exist as separate, discrete molecules. The chemical formulas for covalent compounds are referred to as molecular formulas A chemical formula for a covalent compound.


 0 kommentar(er)
0 kommentar(er)
